Speed to the clinic and patient safety are important goals when working to bring novel genetic therapies to patients. To achieve these goals, Homology Medicines decided to invest in internal development and manufacturing operations not long after the Company’s founding, which included the construction of a 25,000 sq ft GMP manufacturing facility adjacent to its headquarters. This allowed for efficient collaboration, integration, and iteration as Homology developed a commercial platform process that supports both gene therapy and gene editing constructs. By focusing on early product engagement with research, a dedicated process development team, and a collaborative feedback loop with its internal manufacturing facility, Homology established the perfect trifecta for rapid learning.
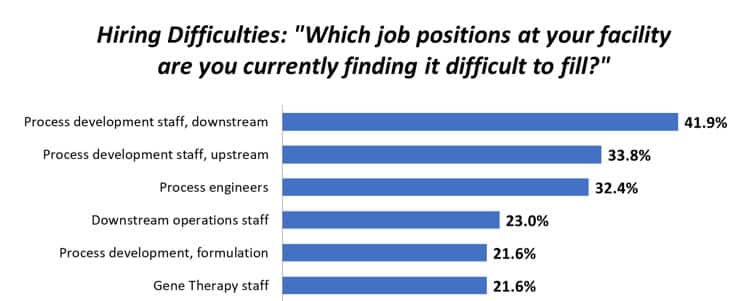
When a pipeline candidate is nominated at Homology, Process Development initially works on assessing a new therapeutic candidate’s target product profile within the established platform process and determines what development enhancement opportunities need to be prioritized to transfer programs into Manufacturing (Figure 1: Fast Track Process Development to Manufacturing Roadmap). It is Homology’s investment in creating a “plug-and-play” platform that promotes fast assessment of development opportunities for each new product candidate which has minimized the time needed to develop novel production and purification processes per product. This plug-and-play platform has afforded Process Development and Manufacturing opportunities to attain comprehensive knowledge by conducting development studies at the 2L scale through the 2000L scale. Process development can now leverage the established platform with new candidates so that resources can be focused on process optimization for each product and supporting a successful technology transfer internally and to CDMO partners.

Figure 1: Fast Track Process Development to Manufacturing Roadmap (Homology)
The interface between Process Development and Manufacturing has been instrumental to the success of the product supply for Homology’s pipeline. The relationship between these two groups has enabled scale-specific collaboration where colleagues from Process Development are able to work continuously and in real-time with Manufacturing to ensure that the process is successfully transferred and executed. It has promoted ownership over the process for all involved as Process Development subject matter experts are able to convey all information related to the development of the processes being transferred in the plant, and Manufacturing colleagues are able to communicate technical and operational improvements in real time. This is where some of our most important rapid learning and employee development happens, and is not similarly achievable with CDMOs. The main benefit of internal manufacturing is that communication and logistical hurdles are minimized because the right people are in the right place together at the right time. This human element of technology transfer and Manufacturing execution enables Homology to successfully deliver its clinical supply for the pheNIX trial.
We have invested in and incorporated single-use technologies, leveraging knowledge from colleagues who worked on the development of the first FDA-approved single-use bioreactor system, to enable a multi-product GMP Manufacturing facility with rapid product change-over. To support these efforts, all single-use components are evaluated and utilized in Process Development before using them in Manufacturing. Working with these technologies in both environments promotes faster scale-up and changeover efforts that have resulted in a successful multi-product facility. Early adoption of these technologies and the collaborative relationship between Process Development and Internal Manufacturing has helped position Homology as a leader in AAV manufacturing.
Looking ahead toward commercial production, we have been able to successfully demonstrate 2000L bioreactor production in our HEK293 transfection platform across multiple product candidates (Figure 2: 2000L Single-Use Bioreactor at Homology Medicines in Bedford, MA Facility). This was a huge accomplishment, first achieved in 2019, and will enable future supply for our therapeutics. The speed at which this was executed would not have been possible without the fast-track process development to manufacturing roadmap that Homology has pioneered and utilized.

Figure 2: 2000L Single-Use Bioreactor at Homology Medicines in Bedford, MA Facility
Early investment in internal development and manufacturing capabilities create the opportunity to boost collaborative efforts across an organization, encourage rapid development of pipeline programs headed for the clinic, inform platform development efforts through an iterative approach at-scale, and perform proactive scale-up studies with processes. These benefits have been realized at Homology through the establishment of a platform process, rapid candidate assessment in this process, collaboration between Process Development and GMP Manufacturing, and has culminated in the successful scale up of multiple products to 2000L starting in 2019. Homology will continue to build our pipeline and leverage our plug-and-play manufacturing platform to support commercial production.