The impact of the Covid-19 pandemic has highlighted the need for a fast and effective vaccine development process. ‘How the Vaccine Industry and Biotechs Can Accelerate Pandemic Responses’ discusses various technologies and approaches that could drive the vaccine industry forward, helping it to tackle ongoing and future pandemics.
New and improved vaccine platforms will enhance the tools at the disposal of vaccine developers. mRNA is emerging as a powerful vaccine platform, while viral vectors were established as a vaccine platform in response to the 2014 Ebola outbreak. Although there are benefits to both technologies, they also have areas for improvement.
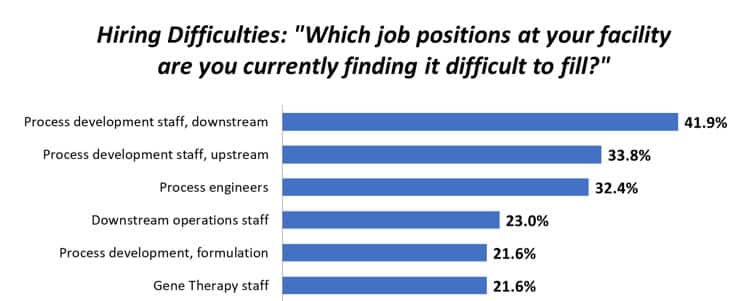
Stringent safety regulations are a bottleneck during vaccine development, of which biosafety testing is an important part. New platforms are being developed that offer faster and more sensitive testing, which could help vaccine developers to reach the clinic sooner.
Collaborating with biotechs is encouraged to make use of their creativity and scientific expertise. In return, the Life Science industry can support biotechs with access to technologies that accelerate timelines and guidance as they interact with authorities to obtain regulatory approval.
Experts at