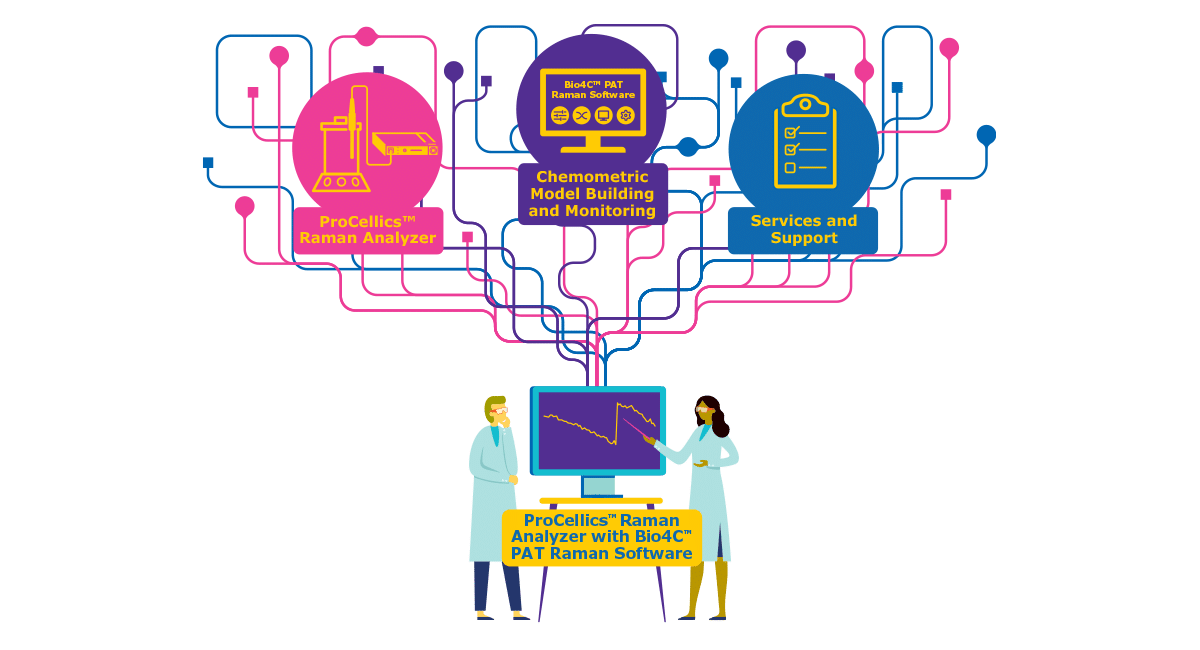
Monitoring critical cell culture parameters is crucial for ensuring the success of cell culture processes. While traditional methods focus on a few parameters like temperature, pH, and dissolved oxygen, they provide only limited insights into the process and cell growth. In contrast, measuring critical process parameters (CPPs) such as glucose, lactate, or ammonium, as well as key performance indicators (KPIs) like total cell density (TCD) and viable cell density (VCD), provides a clearer indication of the status of the culture.
However, measuring these parameters can result in delayed process control and increased contamination risks due to manual sampling. To address these challenges, implementing process analytical technology (PAT) for, in-line, real-time measurements is essential. This method allows for enhanced process control, reduced process risk, and a better understanding of the overall process.
Real-time measurements using Raman spectroscopy allow for more proactive adjustments, reducing the likelihood of batch failures and improving overall process robustness. By leveraging Raman spectroscopy as a PAT tool, cell culture processes can be navigated with greater precision and efficiency, ultimately leading to higher quality and more consistent outcomes.
In conclusion, integrating Raman spectroscopy for real-time measurements of critical cell culture parameters is a pivotal step towards ensuring the reliability and success of cell culture processes. This approach not only minimizes the risks associated with manual sampling but also fosters a deeper understanding of the processes, ultimately contributing to improved process control and more favorable outcomes
To learn more about real-time monitoring of bioprocesses, read our technical article here.