Emerging Biotech
How Process Monitoring Tunnels Ensure Product Quality and Improve Process Performance
With a process monitoring tunnel you can monitor 100s of CPPs in a single graphical interface that captures the overall health – both past and present – of your bioprocess and reliably predicts its future health.
by Abhilash Mohan
Breaking News
Welcome to Emerging BioTalk!
Here, innovators like you will have access to technical and thought leadership content from experts you can trust to help you accelerate your path to commercialization.
As a member of this community, we invite you to share your voice and contribute a blog post of your own by visiting our Submit A Blog Post page.
Finally, to be informed of the latest news and announcements, we invite you to subscribe by clicking “subscribe to our blog” just below.
Thank you for visiting our blog, and we hope to see you again soon!
Welcome to Emerging BioTalk!
Here, innovators like you will have access to technical and thought leadership content from experts you can trust to help you accelerate your path to commercialization.
As a member of this community, we invite you to share your voice and contribute a blog post of your own by visiting our Submit A Blog Post page.
Finally, to be informed of the latest news and announcements, we invite you to subscribe by clicking “subscribe to our blog” just below.
Thank you for visiting our blog, and we hope to see you again soon!
Apply now! 2024 North America Advance Biotech Grant Program
Apply to our Advance Biotech Grant Program and get a chance to benefit from products, services, and consultations from experts. Applications are open until April 30th, 2024.
by Amandine Biehler
How Process Monitoring Tunnels Ensure Product Quality and Improve Process Performance
With a process monitoring tunnel you can monitor 100s of CPPs in a single graphical interface that captures the overall health – both past and present – of your bioprocess and reliably predicts its future health.
by Abhilash Mohan
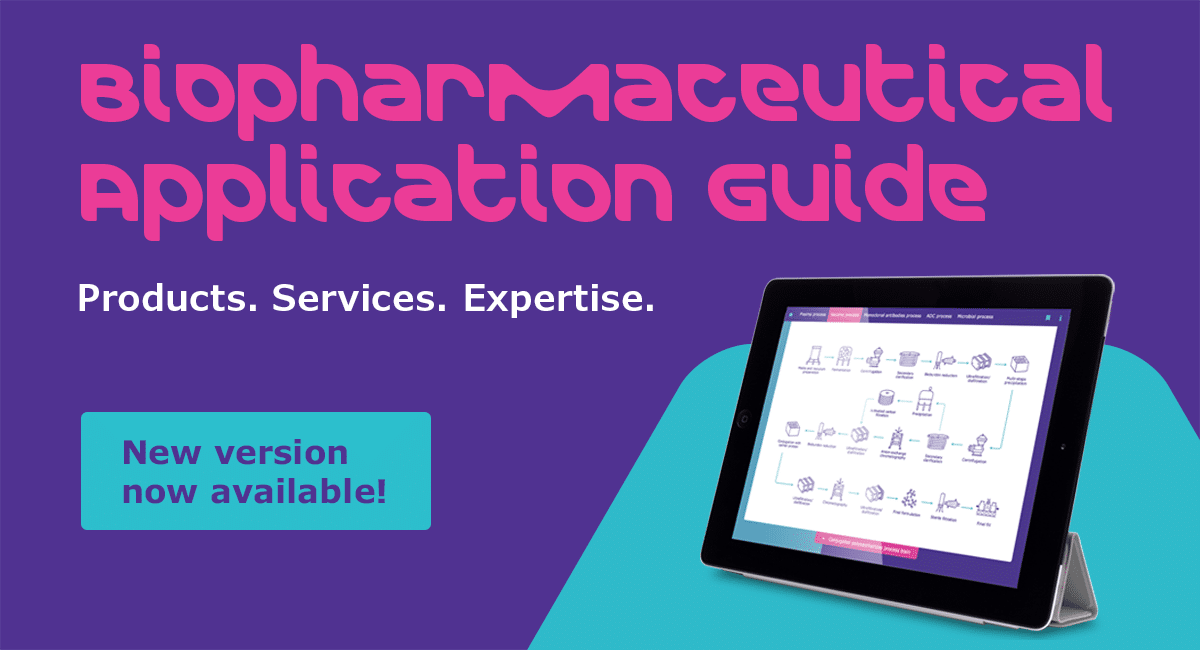
Biopharmaceutical Application Guide helps overcome process challenges
Take a few minutes and navigate through our mAb, ADC, mRNA, Vaccine, and Cell and Gene Therapy process templates. Go ahead and dive into each unit operation to uncover the best option for your process.
by Laurence Carudel
Keys to Success: Allogeneic Cell Therapy Manufacturing
Robust commercial scale bioprocessing workflows must be established along with strategies for sterility assurance during the production of allogeneic products.
Bioprocessing considerations include:
• Establishing a robust process
• Ensuring process compatibility
• Creating the sampling plan
• Planning for media production
Sterility assurance considerations include:
• Using GMP materials
• Qualifying raw materials
• Adopting closed processing
• Performing aseptic process simulations
• Ensuring proper sterile filtration
• Understanding extractables, leachables, and particulates
by Amandine Biehler
Optimizing AAV Manufacturing: Process Intensification with High Salt Lysis and Salt Tolerant Endonuclease
The AAV vector manufacturing process involves cell lysis and nuclease treatment. Advanced techniques, such as using high salt concentration and a salt-tolerant endonuclease, can enhance vector titer and infectivity. Additionally, the development of a non-animal origin, salt-tolerant Benzonase® endonuclease has shown significant impact on AAV5 capsid titers and AAV2 vector infectivity.
by Amandine Biehler
Watch Now! Principles and Lessons Learned from the Benzonase® Success Story
In this webinar, you will learn:
• Key principles of Business Continuity Management
• Best practices for a robust supply chain
• How it translates into success stories, like with the Benzonase® family.
by Amandine Biehler
mAbs
Taking the Lead: Establishing the First GMP Guideline for Cell Culture Media
While cell culture media products play a critical role in the production of many types of therapeutics, there were no specific regulations or GMP certifications governing their use.
• Historically, cell culture media producers used ISO 9001, ISO 13485, and 21 CFR Part 820 to demonstrate product quality and consistency.
• A review of existing standards (ANSI, NSF, ICH, CFR, IPEC-PQG) revealed they were not applicable to cell culture media production processes.
• Merck KGaA, Darmstadt, Germany collaborated with EXCiPACT and industry experts to create a GMP standard for cell culture media, applying the EXCiPACT GMP excipient standard to pharmaceutical auxiliary materials (PAMs), which include cell culture media.
by Amandine Biehler
In-Line Real-Time Monitoring of CHO Cell Culture Process Parameters using Raman Spectroscopy
• Traditional cell culture monitoring offers limited insights, while real-time measurement of critical process parameters provides direct indications of culture state.
• Implementing process analytical technology (PAT) for in-line, real-time measurements improves process understanding, decreases risk, and enables more advanced process control.
by Vincent Gensbittel
Integrated Glucose Control in CHO Culture via Raman Spectroscopy
• Integration of Raman spectroscopy with an automated feedback loop revolutionizes biopharmaceutical manufacturing, enabling real-time glucose level monitoring and precise maintenance for optimal process quality and glycosylation.
• This innovative approach eliminates manual sampling, reduces contamination risks, and ensures reliable, real-time process control, empowering users to navigate upstream biopharmaceutical manufacturing with confidence.
by Vincent Gensbittel
How Process Monitoring Tunnels Ensure Product Quality and Improve Process Performance
With a process monitoring tunnel you can monitor 100s of CPPs in a single graphical interface that captures the overall health – both past and present – of your bioprocess and reliably predicts its future health.
by Abhilash Mohan
Biopharmaceutical Application Guide helps overcome process challenges
Take a few minutes and navigate through our mAb, ADC, mRNA, Vaccine, and Cell and Gene Therapy process templates. Go ahead and dive into each unit operation to uncover the best option for your process.
by Laurence Carudel
Vaccines Technology Transfer: the foundation for a new era of vaccine production
This whitepaper provides an overview of the technology transfer process and describes the development and optimization of a platform to manufacture a vaccine for tropical diseases.
by Carole Inglevert
Supply Spotlight
Coming Full Circle with Biopharma Recycling
This post discusses the environmental challenges posed by the Biopharmaceutical industry’s heavy reliance on single-use systems and the resulting plastic waste. It highlights the industry’s quest for sustainable solutions through advanced recycling methods to achieve circularity.
by Lynn Stephenson, PhD
Climate Neutrality in the Biopharma Industry, How Do We Get There?
To achieve climate target goals, Life Science companies need to put sustainability at the front of business decisions.
by Lynn Stephenson, PhD
Can we change the Biopharma Supply Chain Game so that Everyone Gets a Trophy?
There is a popular board game based on a global disease outbreak that is generally rated 5 stars. As for the real Covid-19 pandemic, it would be hard to find a single person in the Biopharma industry who would give the supply chain performance during the pandemic that kind of high review.
by Lynn Stephenson, PhD
Vaccines
How Process Monitoring Tunnels Ensure Product Quality and Improve Process Performance
With a process monitoring tunnel you can monitor 100s of CPPs in a single graphical interface that captures the overall health – both past and present – of your bioprocess and reliably predicts its future health.
by Abhilash Mohan
Biopharmaceutical Application Guide helps overcome process challenges
Take a few minutes and navigate through our mAb, ADC, mRNA, Vaccine, and Cell and Gene Therapy process templates. Go ahead and dive into each unit operation to uncover the best option for your process.
by Laurence Carudel
Vaccines Technology Transfer: the foundation for a new era of vaccine production
This whitepaper provides an overview of the technology transfer process and describes the development and optimization of a platform to manufacture a vaccine for tropical diseases.
by Carole Inglevert
Taking the Lead: Establishing the First GMP Guideline for Cell Culture Media
While cell culture media products play a critical role in the production of many types of therapeutics, there were no specific regulations or GMP certifications governing their use.
• Historically, cell culture media producers used ISO 9001, ISO 13485, and 21 CFR Part 820 to demonstrate product quality and consistency.
• A review of existing standards (ANSI, NSF, ICH, CFR, IPEC-PQG) revealed they were not applicable to cell culture media production processes.
• Merck KGaA, Darmstadt, Germany collaborated with EXCiPACT and industry experts to create a GMP standard for cell culture media, applying the EXCiPACT GMP excipient standard to pharmaceutical auxiliary materials (PAMs), which include cell culture media.
by Amandine Biehler
Keys to Success: Allogeneic Cell Therapy Manufacturing
Robust commercial scale bioprocessing workflows must be established along with strategies for sterility assurance during the production of allogeneic products.
Bioprocessing considerations include:
• Establishing a robust process
• Ensuring process compatibility
• Creating the sampling plan
• Planning for media production
Sterility assurance considerations include:
• Using GMP materials
• Qualifying raw materials
• Adopting closed processing
• Performing aseptic process simulations
• Ensuring proper sterile filtration
• Understanding extractables, leachables, and particulates
by Amandine Biehler
In-Line Real-Time Monitoring of CHO Cell Culture Process Parameters using Raman Spectroscopy
• Traditional cell culture monitoring offers limited insights, while real-time measurement of critical process parameters provides direct indications of culture state.
• Implementing process analytical technology (PAT) for in-line, real-time measurements improves process understanding, decreases risk, and enables more advanced process control.