The success of cell and gene therapy products in recent years to treat a host of complex diseases has made production that much more critical. Many companies have begun to ramp up production timelines, so as to meet patients’ needs, while still producing efficacious and safe cell and gene therapy materials. However, many biomanufacturers have found themselves in a bit of a quandary: How do we traverse the turbulent regulatory waters governing the production of these novel products? Moreover, how has the COVID-19 pandemic affected the regulatory process? Thankfully, the experienced team at MilliporeSigma has surveyed the landscape and can offer some insight into these critical issues. In this GENcast, we chatted with two team members and picked their brains about some of the challenges currently facing manufacturers in this space. Listen in and hear what they have to say…
- Jessica Hoganhttps://emergingbiotalk.com/author/jessica-hogan/April 19, 2023
- Jessica Hoganhttps://emergingbiotalk.com/author/jessica-hogan/April 12, 2023
- Jessica Hoganhttps://emergingbiotalk.com/author/jessica-hogan/April 5, 2023
- Jessica Hoganhttps://emergingbiotalk.com/author/jessica-hogan/
Related Posts
Emerging Biotech
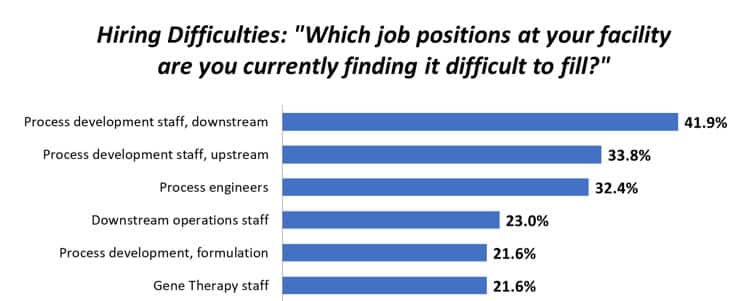
Hiring Challenges in Bioprocessing for Start-ups
Creating Bottlenecks in Europe; Opportunities for Production Staff BioPlan Associates, Inc. Hiring of bioprocessing professionals has remained a…
by Eric Langer May 13, 2020
Emerging Biotech
When to sprint and when to breathe in the race from lab to clinic – 5 critical steps to success
As an early-stage or small-sized biotech company, you need to move fast and be nimble. Success is measured…
by Christian Cattaruzza May 13, 2020
Emerging Biotech · Gene Therapy
Starting your journey into manufacturing – choosing a cell line development provider
One of the biggest decisions which any company developing biological medicines makes is to move forward to the manufacture of their product. …
by Dr. Jonathan H. Dempsey August 27, 2020
Emerging Biotech
A Streamlined Approach to Analytical Method Development and Implementation
The development and implementation of analytical methods are essential for success at all phases of a molecule’s journey,…