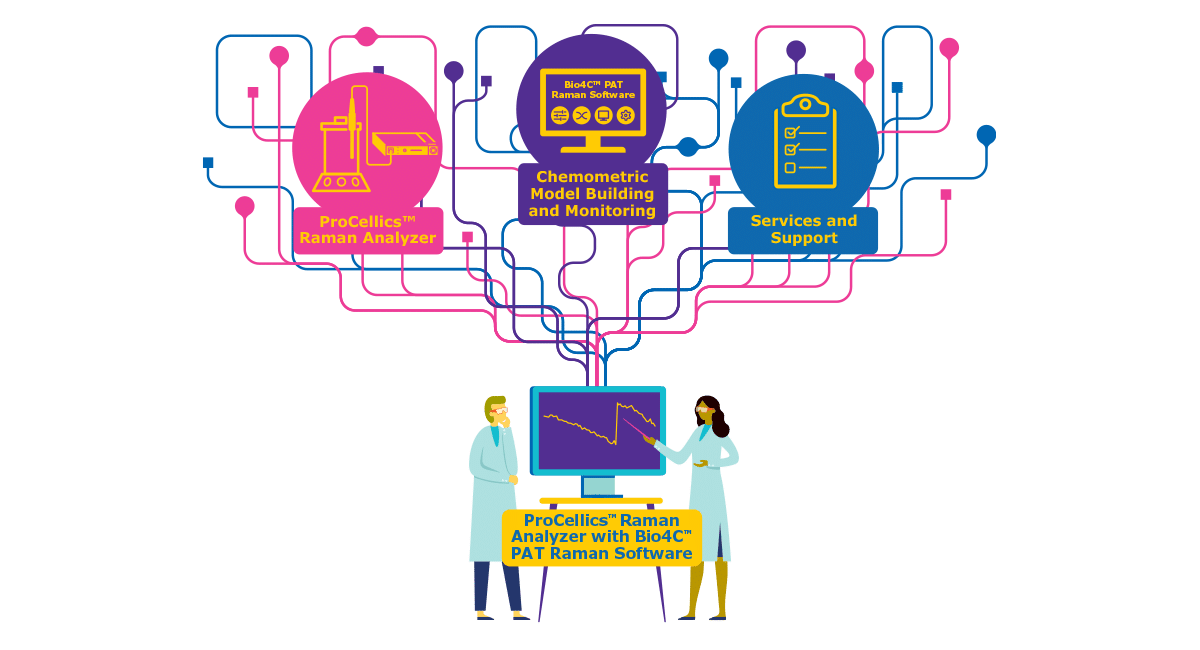
Gene therapies hold the promise to change lives. As your path to patients accelerates, how can you assure the robust process design, intensification and scalability that meets your evolving manufacturing needs? And what benefits can a templated process bring to your commercial success?
Learn the answers to these questions and more in this webinar.